Our Network
Through our global Aspen API network, we ensure flexibility and delivery reliability

Location Sioux City, Iowa, USA
In our facility in Sioux City (Iowa, USA), we collect porcine mucosa and produce a crude heparin, that is then transported to our facilities in Oss for final purification and product release. Having this Sioux City based facility in an important livestock area in the USA, provides close access to many large slaughterhouses, and thus to large mucosa quantities. Large-scale mucosa collection is indispensable to be able to continuously serve the world market needs for heparin sodium.
Inspection approvals
US FDA
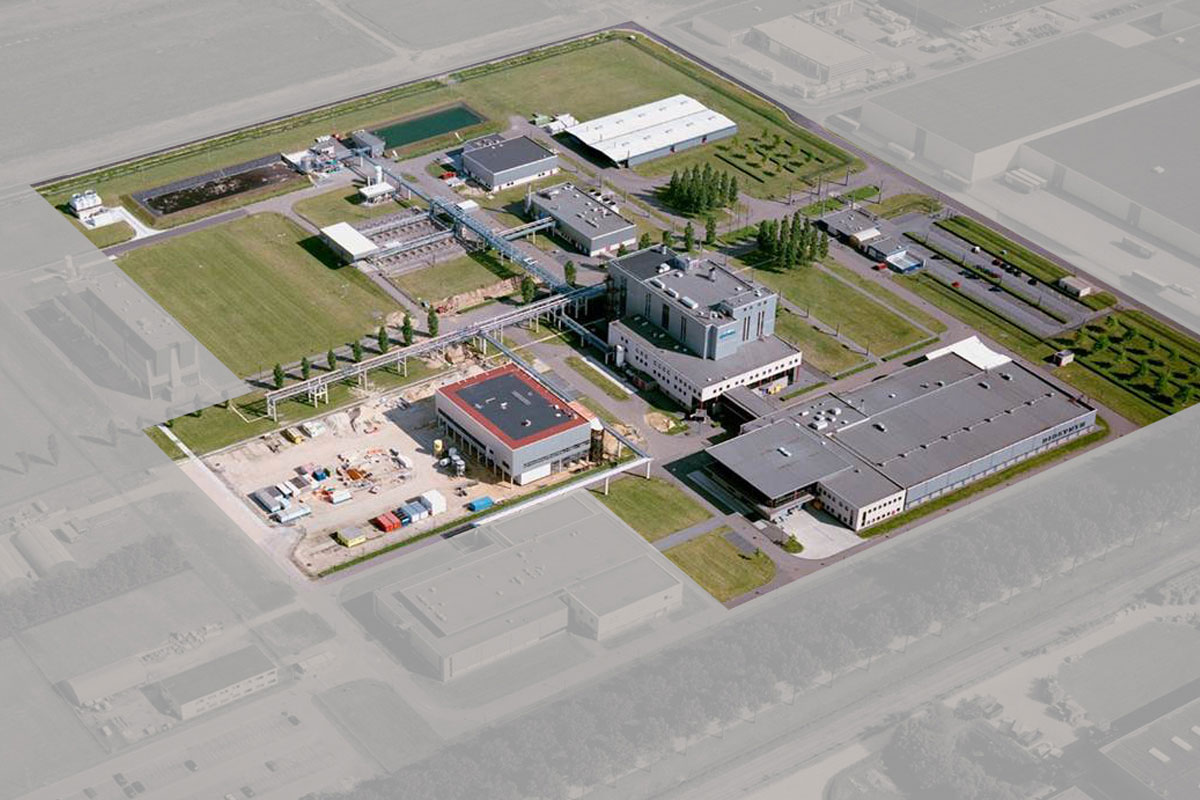
Location De Geer, Oss, The Netherlands
The Diosite (‘De Geer’) facility is dedicated to chemical manufacturing and contains a modern, computer-directed, multi-purpose factory used for larger production campaigns with reaction vessels up to a volume of 10,000 liters.
Also located at this site are the sieving, milling and micronisation of the APIs, as well as the central warehousing and distribution operations.
Inspection approvals
ANVISA, Dutch HA, KFDA, PMDA, Russian HA, Turkish HA, US FDA.

Location Moleneind, Oss, The Netherlands
The Moleneind site is located in the heart of Oss. Numerous activities are performed on site, including peptide manufacturing, biochemical manufacturing and small-scale chemical manufacturing.
Also our Process and Analytical Development, our stability chambers, Quality Control and Quality Assurance departments are located at Moleneind.
Inspection approvals
ANVISA, Dutch HA, KFDA, PMDA, Russian HA, Turkish HA, US FDA.

Fine Chemicals Corporation, Cape Town, South Africa
Fine Chemicals Corporation, a wholly owned Aspen subsidiary, is located in Cape Town, South Africa.
The production facilities at FCC are divided into ten production buildings and the total production processing area under roof inclusive of mezzanines amounts to 6965 m2. The raw materials and finished goods stores comprise a further 3388 m2. The total site area in 2,7 ha / 27000 m2.
Inspection approvals
US FDA, PMDA, TGA, SAHPRA, EDQM/ANSM

Location Boxtel, The Netherlands
Aspen API runs a biochemical operation in Boxtel. In this dedicated facility, urine from pregnant women is collected and further processed into the final API Chorionic Gonadotrophin (HCG).
In 2018 this facility has undergone a major renovation including building a modern purification suite and the introduction of a virus filtration step, thereby meeting worldwide regulatory requirements.
Inspection approvals
Dutch HA, PMDA, Russian HA, Turkish HA, US FDA.


 Australia
Australia France
France Germany
Germany Netherlands
Netherlands Russia
Russia China
China Japan
Japan Mexico
Mexico Malaysia
Malaysia Philippines
Philippines Taiwan
Taiwan Ghana-Kama
Ghana-Kama Kenya-Beta Healthcare
Kenya-Beta Healthcare Mauritius
Mauritius Nigeria
Nigeria South Africa-Commercial
South Africa-Commercial Tanzania-Shelys
Tanzania-Shelys Canada
Canada
 Ghana-Kama
Ghana-Kama  Kenya-Beta Healthcare
Kenya-Beta Healthcare  MAURITIUS
MAURITIUS  South Africa-FCC
South Africa-FCC  South Africa-HOLDINGS
South Africa-HOLDINGS  South Africa-Pharmacare
South Africa-Pharmacare  Tanzania-Shelys
Tanzania-Shelys  Canada
Canada  China
China  Malaysia
Malaysia  Philippines
Philippines  France
France  Germany
Germany  Netherlands
Netherlands  Netherlands-Aspen API
Netherlands-Aspen API  Russia
Russia